

| Urinary incontinence,
the unintentional loss of urine, is estimated to affect approximately 13
million people in the United States, 85% of them women. Although caused
by a variety of factors, women are most likely to develop incontinence either
during or following pregnancy or childbirth, or after the hormonal changes
of menopause, which cause weakened pelvic muscles. Older men often become
incontinent as the result of prostate surgery.
PPTI is focused on the development of an urethral bulking agent to treat female stress urinary incontinence. However, the same technology is also believed to be applicable to the treatment of male incontinence and the treatment of a condition termed vesico-ureteral reflux, where urine flows backwards from the bladder to the kidneys with adverse consequences. |

|
Urinary incontinence can lead to lifestyle restrictions, depression, and may also lead to complications such as urinary tract infections and skin rashes. It is estimated that over $16 billion is spent in the U.S. every year on incontinence-related care. Stress urinary incontinence is the loss of urine from pressure on the bladder occurring during exercise, coughing, sneezing, etc. This may be due to abnormal movement of the bladder and urethra and/or a weakening of the urethral sphincter, a ring-shaped muscle at the base of the bladder that controls urine flow. Normally, the sphincter is able to withstand incremental increases in bladder and abdominal pressure until the individual decides to void; a weakened sphincter lacks this capability. PPTI is developing an injectable product based on one of its patented silk-elastin polymers for use as a urethral bulking agent (UBA) for the treatment of female stress urinary incontinence (SUI). Once injected into the tissues surrounding the sphincter, the material changes from a polymer solution transforms to a firm yet pliable hydrogel. In an office-based procedure, the physician will inject PPTI's UBA into the urethral tissue just below the base of the bladder using a standard cystoscope. The polymer solution initially spreads through the targeted area, causing the tissue to expand. At body temperature, the polymer molecules then bind to each other within a matter of minutes, trapping the surrounding water and forming the hydrogel. The increased "bulk" at the injection site provides the sphincter muscles with additional capability to control the release of urine. An important property of the product is that the volume of material injected remains constant in the liquid to gel transition. Thus, the tissue expansion observed by the physician upon administration of the product should be subsequently maintained. 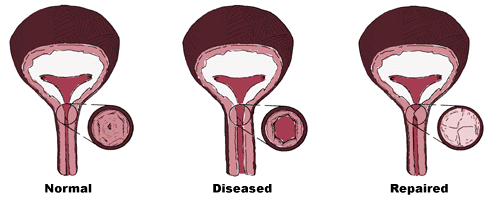 Women suffering from SUI may opt to use absorbent pads or externally applied urethral plugs or caps, which are inconvenient and do not directly address the cause of the problem. Exercises to strengthen the pelvic muscles may also be tried, but are typically not very effective over time due to the constant effort required. Currently, there are two different curative treatment options available in the U.S., surgery and Contigen® Bard® Collagen Implant - an injectable urethral bulking agent. Surgery has a relatively high level of efficacy and is considered very durable, but is invasive with the associated risks and recovery time. Contigen® is only moderately effective and has not proven to be very durable. Because the bovine collagen used to make Contigen® is fairly rapidly degraded by the body and since the product is a suspension of collagen particles in water, it loses volume over time and thus usually requires multiple injection sessions to achieve and maintain continence. Additionally, approximately 3% of the U.S. population develop allergic reactions to bovine collagen. Therefore, prospective patients must be prescreened using a skin test before the product can be administered. A number of companies are reported to be developing urethral bulking agents for the treatment of SUI in the U.S. Several involve the testing of products sold outside the U.S. in order to obtain FDA approval. PPTI believes its product is distinguished from other bulking agents in several important ways. The majority of competing technologies are suspensions or slurries of solid particles (such as collagen, glass, silicone, teflon) in an aqueous carrier such as saline. When injected, the carrier liquid dissipates through the tissues with time, usually within 24 hours, such that the effective bulking volume may be lost. This requires the physician to either overcompensate for the expected volume reduction upon initial administration, with increased risks to the patient, or to "top off" the bulking effect with repeated administrations of the product over time, substantially increasing the cost of treatment. Particle based products are also potentially subject to migration from the injection site. Other hydrogel technologies of which the Company is aware are either preformed gels, difficult to administer by injection, or polymer solutions mixed with a chemical cross-linking agent prior to injection. PPTI believes that products based on such technologies are limited in their overall performance including durability, biocompatibility and ease of use. Products based on yet other technologies, such as the implantation of balloons (subsequently expanded with a liquid) or fibers, require more invasive administration procedures. PPTI's UBA product is currently in preclinical testing to obtain the data needed to support an Investigational Device Exemption (IDE) submission to the FDA for approval to begin human clinical testing. The Company expects to file the IDE before the end of 1998. Prior feasibility studies have shown the product to be biocompatible, non-immunogenic, resistant to migration, and more durable than collagen-based products. |