


| In 1996, there were 3.2 million cosmetic procedures performed in the U.S. according to the American Academy of Cosmetic Surgery. The #1 reason people, both male and female, have such procedures done is to improve their self-image.
This is not surprising given the tens of billions of dollars spent on beauty care products each year in the U.S. alone. A significant portion of these products are for skin care, used to protect and moisturize the skin, in the hope that the unavoidable effects of aging and exposure to the environment ("wrinkles") can be minimized or deferred. Ultimately, many people decide to seek the services of a dermatologist or a cosmetic or plastic surgeon to have a cosmetic procedure done. These procedures range from the traditional face or brow lifts (about 75,000 U.S. procedures); to dermabrasion, chemical peels, or laser resurfacing (about 750,000 U.S. procedures); to retin-A treatments (about 750,000 U.S. procedures). Factors involved in deciding upon a course of treatment include the patient's goals, the expense, the invasiveness of the procedure, the recovery time involved, and potential adverse consequences. |

|
One of the more popular procedures in the last decade has been the injection of bovine collagen, used to correct dermal "contour deficiencies" (frown lines, worry lines, wrinkles, crow's feet, facial scars, marionette lines, etc.). Today, several other materials are used in a similar manner. The procedure appeals to people because it provides a noticeable benefit with essentially no recovery time and minimal risk of adverse effects. All that is required is a quick visit to the physician's office, with immediate resumption of normal activities. In 1996, there were about 325,000 U.S. procedures involving such products. With the aging of the U.S. population and the introduction of superior products, the market is expected to grow significantly. PPTI is developing an injectable dermal augmentation product for the treatment of contour deficiencies due to aging and environmental exposure, disease such as acne and cancer, and surgery. The product is based on one of the Company's patented silk-elastin polymers. Once injected into the skin, the material changes from a polymer solution to a firm yet pliable hydrogel. In an office-based procedure, the physician will inject PPTI's product into or under the skin using a very fine hypodermic needle. The polymer solution initially spreads through the targeted area, causing the tissue to expand. At body temperature, the polymer molecules then bind to each other within a matter of minutes, trapping the surrounding water and forming the hydrogel. The degree of tissue augmentation at the injection site can be easily adjusted depending on the desired outcome. An important property of the product is that the volume of material injected remains constant in the liquid to gel transition. Thus, the change in tissue dimensions observed by the physician upon administration of the product should be subsequently maintained. 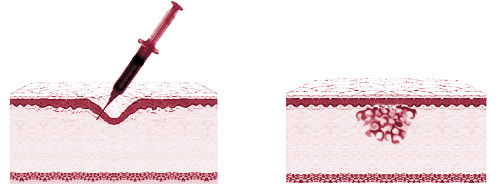 Patients seeking correction of soft tissue contour deficiencies can obtain dramatic improvement through face and brow lifts. However, in comparison with injectable augmentation products, they are expensive, invasive, require relatively long recovery times, and are subject to the same complications as any other surgery. Another surgical option is the implantation of preformed products such as solid silicone implants, Gore Tex®, and human cadaver skin processed to remove the cellular components. However, these are typically used where substantial contour corrections are required. Surface treatments such as dermabrasion, chemical peels, and laser resurfacing also can provide dramatic improvements, but the procedures basically remove the top layers of the skin, with the associated potential for adverse affects and time required for recovery. Many non-invasive treatment options are on the market, but the degree of improvement is typically minimal and regular re-treatments are sometimes required. Injectable dermal augmentation products provide the patient with a minimally invasive option to achieve an immediate reasonable correction of contour deficiencies with little or no recovery period. Current products approved for use in the U.S. include bovine collagen (Zyderm®, Zyplast®) and porcine gelatin (Fibrel). Doctors also sometimes use the patient's own fat. None of these materials are considered very durable, typically lasting less than 3 to 6 months. This is a major drawback in that the average patient who might desire treatment with an injectable dermal augmentation product simply cannot afford to have the procedure repeated over and over to correct the same areas. Several companies are reported to be developing hydrogel-based injectable dermal augmentation products. The technologies of which the Company is aware are either preformed gels, difficult to administer by injection, or polymer solutions mixed with a chemical cross-linking agent prior to injection. PPTI believes that products based on such technologies are limited in their overall performance including durability, biocompatibility and ease of administration. PPTI's dermal augmentation product is currently in preclinical testing to obtain the data needed to support an Investigational Device Exemption (IDE) submission to the FDA for approval to begin human clinical testing. The Company expects to file the IDE during 1999. Prior feasibility studies have shown the product to be biocompatible, non-immunogenic, resistant to migration, and more durable than collagen-based products. |